Call us now:
GraftRX
Strong
Effortlessly launch your business in Ireland, the UK, USA, or EU with our complete formation solutions.

Easy-to-Use

Flexible

Reimbursable
Specialized wound care solutions
We provide a comprehensive line of human allografts for use in surgical specialties such as spine, orthopedics, and wound care. Partnering with top-tier manufacturing facilities, GraftRX ensures that all products are processed with the highest standards of sterility and safety, using certified, fully validated cleanroom environments and strict aseptic techniques.
As experts in specialized wound care solutions, GraftRX delivers innovative options for patients with critical wounds in assisted living facilities, nursing homes, rehab centers, hospices, and home care settings. GraftRX offers tailored solutions to help improve patient outcomes, providing unparalleled support to healthcare providers nationwide. Trust GraftRX for safe, effective, and reliable wound care solutions.
What is Allograft?
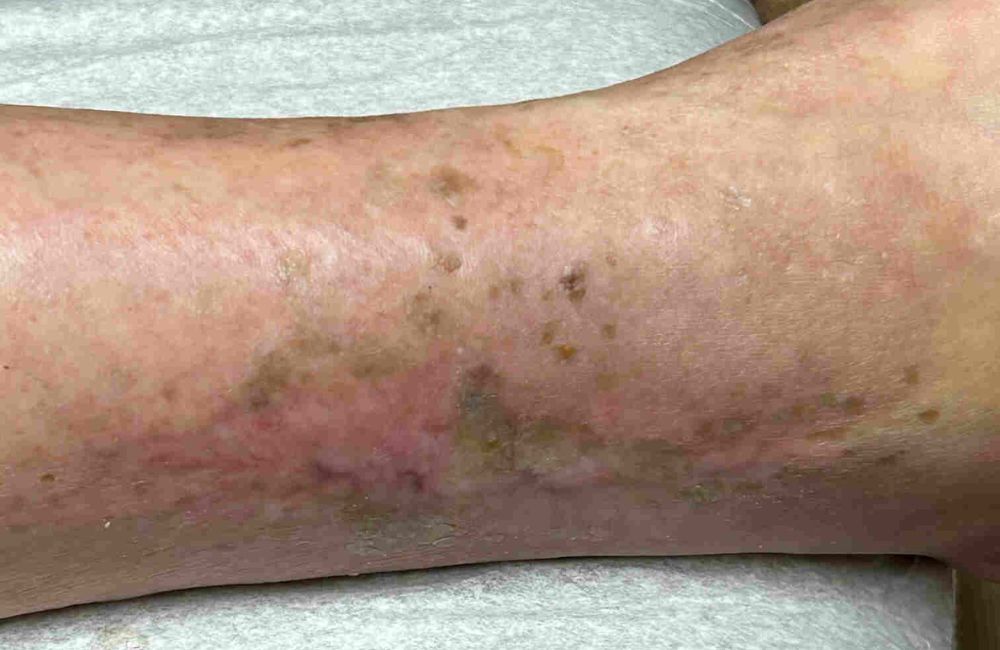
An allograft is donated human tissue transplanted from one individual to another, often used to aid the recipient in regenerating healthy tissue and restoring normal function. Among the most commonly treated wounds with allografts are:
- Diabetic Foot Ulcers (when the wound has shown less than 50% healing after 30 days of treatment),
- Vascular Leg Ulcers (venous and arterial, where the wound has persisted for a minimum of 90 days with at least 30 days of documented treatment),
- Pressure Ulcers (such as sacral decubitus ulcers).
These challenging wound types benefit from the regenerative properties of allograft tissue, promoting faster healing and improved outcomes.
What is Amniotic Tissue for Transplant?
Amniotic tissue grafts are derived from the amniotic sac, which surrounds a baby during pregnancy. Following the planned delivery of healthy babies via Cesarean section, mothers may choose to donate their placenta and the attached amniotic membranes. These membranes undergo a rigorous process of cleaning, processing, drying, cutting into patches, and sterilization to ensure they are safe for patient use. Amniotic membrane allografts have demonstrated the ability to enhance surgical outcomes and wound healing by reducing fibrosis, preventing adhesions, and delivering essential growth factors that support tissue regeneration.

Incredibly versatile wound covering for patients with:
Diabetic Foot Ulcer • Vascular Leg Ulcers • Pressure Ulcers
Advanced amniotic tissue products.
AmnioProPlus
AmnioQuadCore
AmnioTriCore
AmnioCore
AmnioCore Pro
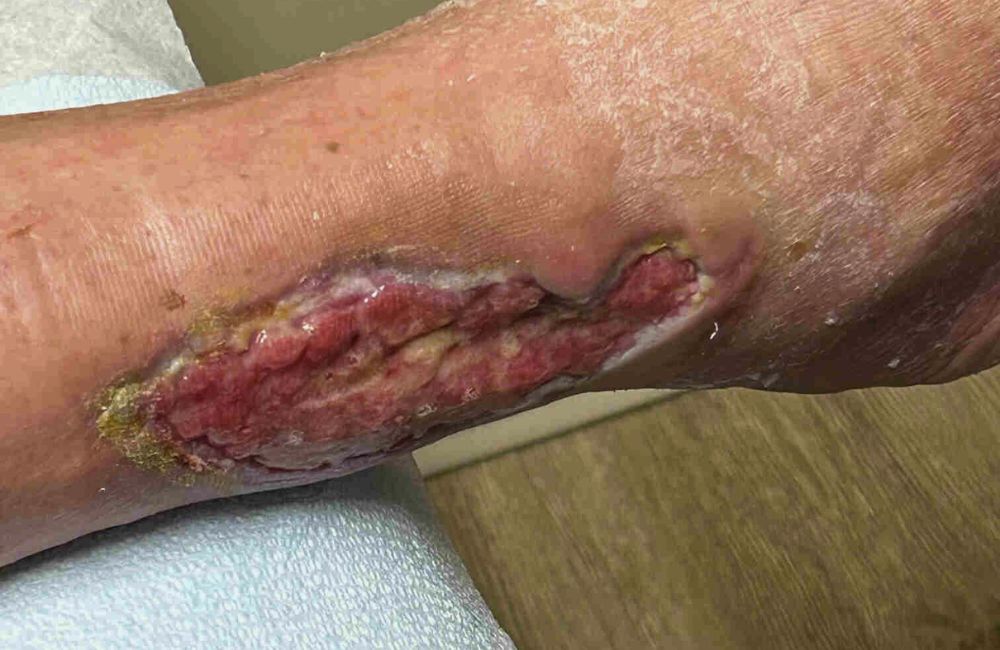
Before